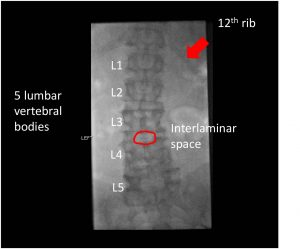
Board Review 1 – Case 7
Neuroradiology board review. This lecture is geared towards the ABR core exam for residents, but it would be useful for review for the ABR certifying exam or certificate of added qualification (CAQ) exam for neuroradiology.
More description and the answer (spoiler!) are seen below the video.
This case is a patient presenting to the ER with headache. The CT demonstrates scattered areas of edema, warranting further evaluation with an MRI.
The MRI shows parenchymal nodular areas of enhancement with surrounding edema. There is also coating of the surfaces of the brain with abnormal enhancement, known as leptomeningeal enhancement. When this is nodular, it is highly concerning for metastatic disease.
The diagnosis is: metastatic disease
Nodular leptomeningeal enhancement on MRI is concerning for several serious pathologies. The differential includes meningitis, both with bacterial and unusual (TB and fungal) pathogens, inflammatory pathologies such as sarcoidosis, and carcinomatosis (as in this case).